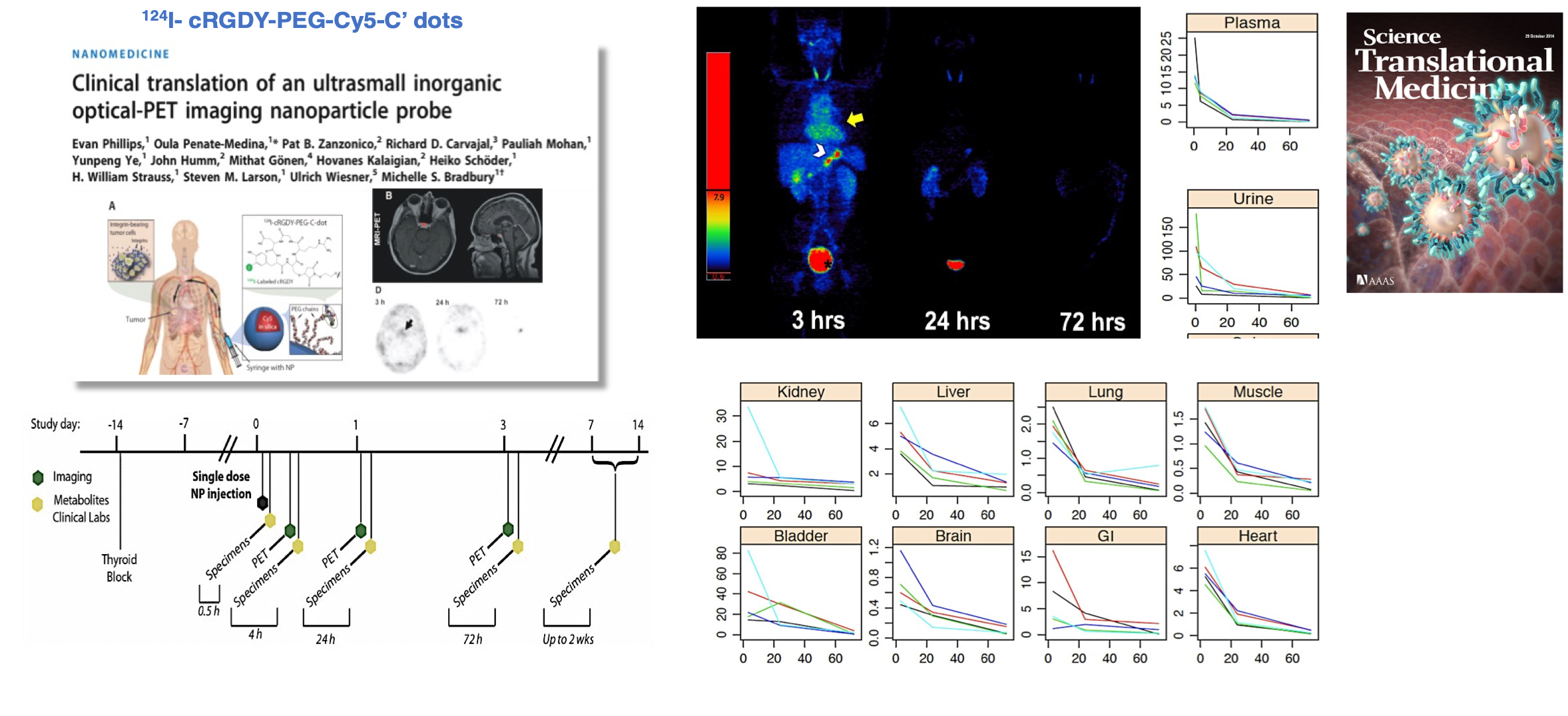
Figure 1. Favorable PET pharmacokinetic data is shown over time using a novel 124I-labeled integrin-targeting particle platform, 124I-cRGDY-PEG-Cy5-C’ dots, that was FDA cleared for a first-in-human clinical trial. A principal aim of this trial was to demonstrate improved product safety of this targeted multimodal platform in metastatic melanoma patients, along with targeted delivery as a secondary objective. C’ dots are ~6 nm hydrodynamic diameter designed for bulk renal clearance as part of a target-or-clear paradigm. E. Phillips, P. Zanzonico, J. Humm, S. Larson, U. Wiesner, M. Bradbury et al. Sci Trans Med 6, 260ra149 (2014)